
News Source: News Agency of BIT
Contributed by: School of Chemistry and Chemical Engineering
Recently, the team of Professor Xu Baohua from BIT has made important progress in constructing poly ionic liquid-silver (PIL-Ag) hybrid materials for the efficient electrocatalysis of carbon dioxide (CO2) to carbon monoxide (CO). The related research results have been published in the top international journal of Chemical Engineering Journal entitled "efficient electrocatalytic reduction of CO2 to CO on highly dispersed Ag nanoparticles confined by poly (ionic liquid)". BIT is the only corresponding author workplace, Professor Xu Baohua from the School of Chemistry and Chemical Engineering is the corresponding author of this paper, and pre-hired assistant professor Duan Guoyi is the first author of this paper.
Electrocatalytic carbon dioxide reduction (CO2RR) technology can realize the conversion and utilization of CO2 under mild conditions, driven by renewable electricity, which is of great significance to achieve the goal of "carbon neutrality". Among all the conversion modes, electrocatalytic CO2-to-CO conversion has attracted the attention of the scientific community because of its advantages of high utilization rate of raw materials, wide application scenarios and low production cost. Developing electrocatalysts with high activity, high selectivity and high stability is an important prerequisite for further promotion of the practical application of this technology. Ag is considered as the most potential active metal because of its moderate activation energy of *COOH intermediate, weak adsorption energy of CO and low activity of hydrogen evolution. Many strategies have been used to improve performance of Ag-based CO2RR materials. Among them, surface modification of Ag can significantly improve the CO selectivity (> 80%), but the bias current density is small (< 50 mA·cm–2), and the research on catalytic stability is less.
In the preliminary work, Professor Xu Baohua's team constructed a series of PIL- monometallic/bimetallic hybrid materials, which realized the electrocatalytic conversion of CO2/CO to C2+ products with high current density, high selectivity and high stability, and revealed the regulation of PIL- metal interface properties on the reaction path and the effect of PIL on the high dispersion of metals (Angew. Chem. Int. Ed. , 2022, 61: e202110657; Appl. Catal. B. Environ. , 2021, 297: 12047; Appl. Catal. B: Environ. , 2022, 313: 121459; Chem. Eng. J., 2023 , 451: 138491; Fundamental Research , 2022, 2: 937) In this work, a series of PIL-Ag hybrid materials were synthesized by introducing Ag salts into PIL by simple impregnation method. PIL has a significant confinement effect on the in-situ generated highly dispersed Ag–Cl species. The cationic skeleton structure and the types of Ag salts in PIL directly affect the formation and evolution of Ag(I) complexes, Ag–Cl clusters and Ag–Cl nanoparticles. Thanks to the highly dispersed imidazole-pyridine-imidazole tridentate chelating sites in PIL and the appropriate Ag+ supply rate, the optimal PIL-Cl@AgOAc-1.0 hybrid material was prepared. In 1 M KOH solution, at the potential of–-0.91 V (relative reversible hydrogen electrode), a high CO Faraday efficiency of 96.8% and a bias current density of 207.0 mA·cm–2 were achieved. In addition, the catalyst maintained a high CO Faraday efficiency (~86%) after continuous electrolysis for 100 hours at 100 mA·cm–2, which showed excellent catalytic stability. The mechanism study shows that although the three Ag–Cl species have similar intrinsic activities, the dispersion form of Ag–Cl species, the number of active sites and the mass transfer of reactants jointly determine the apparent electrocatalytic performance at high reaction rate.

Fig. 1 structural characterization results of pil-cl @ Agoac hybrid materials with different Ag impregnation amounts
The structure shows that the impregnation amount of Ag affects the existing form of Ag–Cl species: Ag(I) complex is the main form at low impregnation amount, Ag–Cl cluster is the main form at medium impregnation amount, and Ag–Cl nanoparticles are the main form at high impregnation amount. TEM XRD reveals that Ag–Cl nanoparticles are rare hexagonal Ag-Cl, which indicates that PIL skeleton structure has confinement effect on the formation of Ag–Cl nanoparticles.
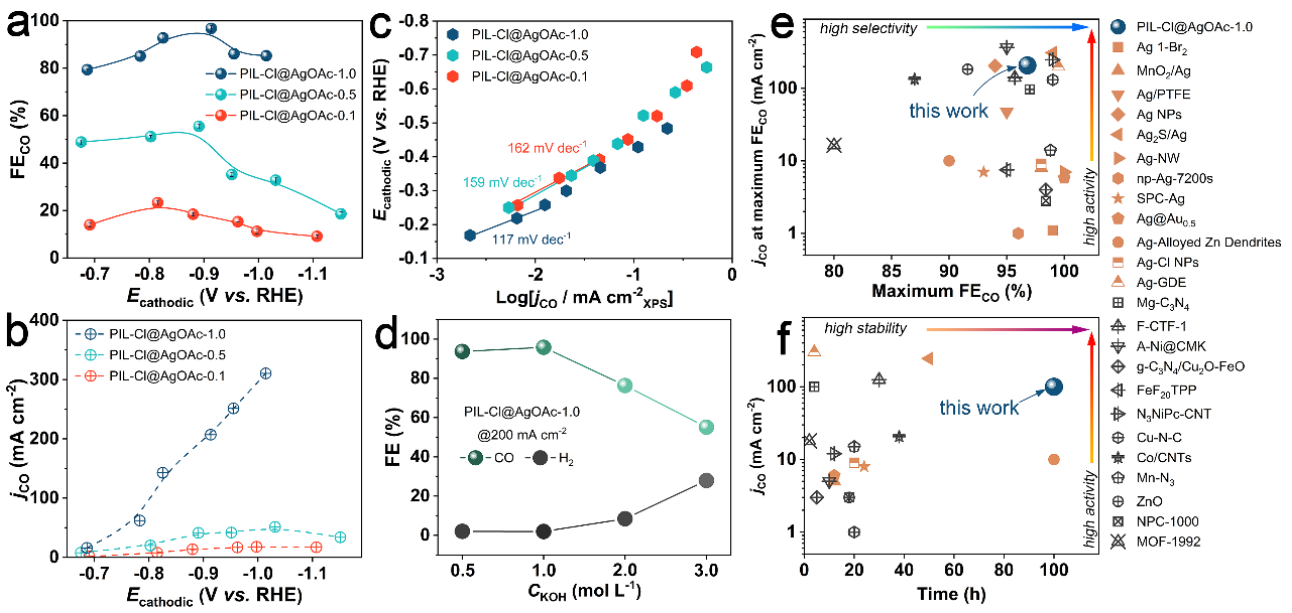
Fig. 2 CO2RR performance of PIL-Cl@AgOAc hybrid materials under different Ag impregnation levels, and the comparison of Faraday efficiency, bias current density and stability of CO produced by PIL-Cl@AgOAc-1.0 with other advanced catalysts reported in the literature.
The performance evaluation shows that different Ag impregnation amount has a significant effect on the selectivity and activity of electrocatalytic CO2 to CO conversion. Kinetic (Tafel) analysis revealed that there was little difference in intrinsic activity of active sites with different impregnation amounts. Compared with other advanced catalysts reported in the literature, the optimal PIL-Cl@AgOAc-1.0 catalyst shows excellent selectivity, activity and catalytic stability. Further research shows that the cationic skeleton structure in PIL and the types of Ag salts affect the existing forms of Ag–Cl species by influencing the interaction strength of PIL-Ag and the supply rate of Ag+. The CO selectivity of Ag–Cl nanoparticles decreases slowly at high current density, and the overall CO selectivity is closely related to the number of Ag–Cl nanoparticles. Ag–Cl clusters show high initial CO selectivity, but it is difficult to maintain high selectivity at high current density.
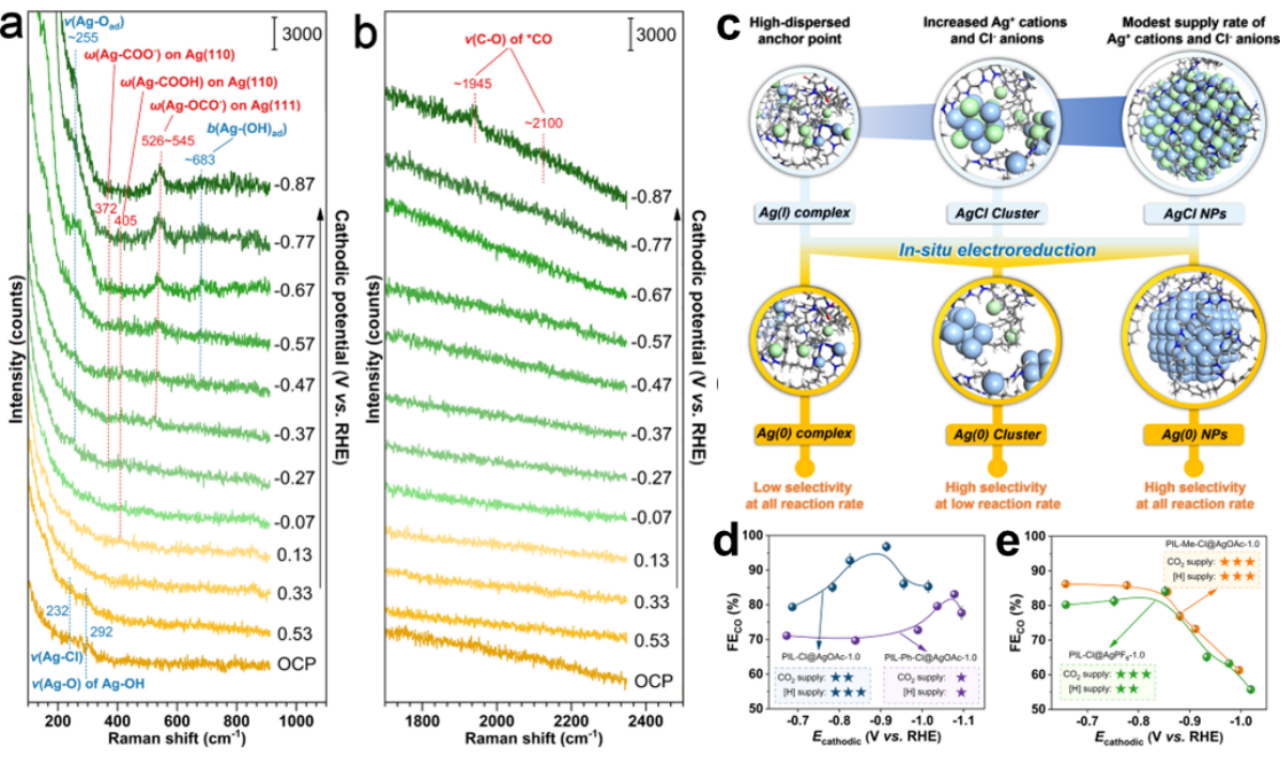
Fig. 3 In-situ Raman spectrum analysis, evolution law of different Ag–Cl species and factors affecting apparent CO2RR performance
In-situ Raman spectroscopy found that after a certain cathodic potential was applied (before CO2RR occurred), all kinds of Ag–Cl species were reduced to metallic Ag(0) species, and these Ag(0) species were considered as the actual active surfaces where CO2RR occurred. The evolution rules of different Ag–Cl species can be summarized as follows: the highly dispersed anchor points provided by PIL ( the abundant chelating sites and dense electrostatic network of PIL), are conducive to the formation of Ag(I) complexes when less Ag salts are introduced; Then, the Ag+ cation diffused at a moderate rate further reacts with Cl– ion in PIL, and the Ag(I) complex is transformed into Ag–Cl cluster. However, the formation of Ag–Cl nanoparticles requires the continuous but slow supply of Ag+ cations to interact with relatively weak PIL-Ag. The apparent CO2RR activity of different hybrid materials at high current density is not only related to the existing form and quantity of Ag active sites, but also closely related to the effective supply of reactants (i.e. CO2 and H2O) caused by different PIL structures and compositions. This work is supported by the National Natural Science Foundation of China's Outstanding Youth Fund and General Program, as well as the Beijing Key Laboratory of Chemical Power Supply and Green Catalysis.
With an introduction to the author:
Xu Baohua, professor, doctoral supervisor, School of Chemistry and Chemical Engineering, mainly engaged in the design and preparation of ionic liquid hybrid materials and the research of new green catalysis process. He has presided over more than 10 general projects of the National Natural Science Foundation of China, outstanding youth fund projects, China Academy of Sciences and enterprise cooperation projects. He has published more than 80 papers including J. Am. Chem. Soc., Angew. Chem. Int. Ed., Appl. Catal. B-Environ., Chem. Eng. J. and Green Chem., ans has applied for and authorized 15 invention patents and 1 PCT patent.
Duan Guoyi, assistant professor of chemistry and chemical engineering college, tutor of master students, has mainly engaged in the research of catalysts and reactors related to heterogeneous electrocatalytic conversion of small energy molecules and participated in a number of national, provincial and ministerial projects and enterprise R&D projects. He has published more than 10 papers in Angew. Chem. Int. Ed., Appl. Catal. B-Environ., chem. eng.j., Prog. Energ. Combust. Sci., J. Clean. Prod., ChemSusChem and other journals.